
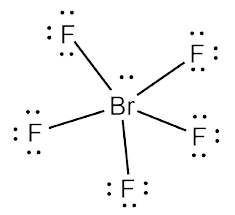
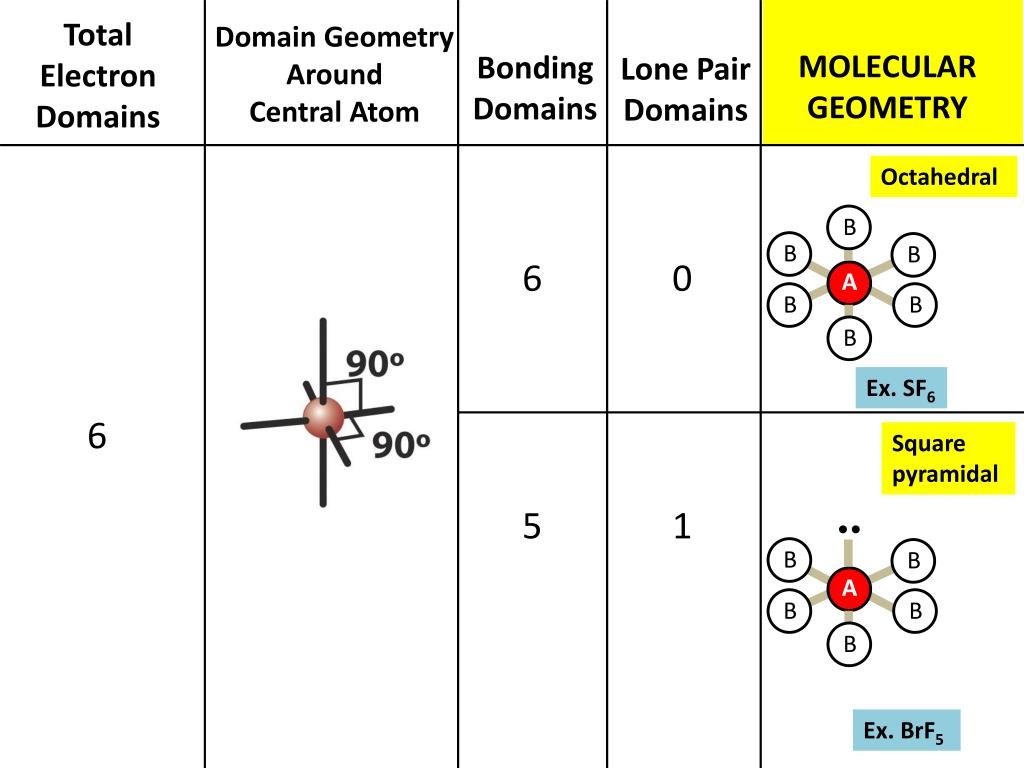
But bond polarity of Br-F is not canceled to. It has a difference in electronegativity values between bromine and fluorine atoms, with fluorine’s pull the electron cloud being greater than bromine’s. One thing to keep in mind while drawing Lewis structure is that the Octet Rule can be violated in these three situations but, we don’t need to think about it each time as it is rare and these exceptions will only occur when necessary.Įxception 1: If there is an odd number of valence electrons like 3,5,7, etc.Įxception 2: If there are very few valence electronsĮxception 3: If there are too many valence electrons The molecule of bromine trifluoride (with trigonal bipyramidal shape BrF3 molecular geometry) is tilted at 86.2 degrees bond angle of F-Br-F. But, as we know, there are no extra electrons. Quiz your students on Lewis Structure For BrF5, Molecular Geometry, Bond Angle, Hybridization, Polar or Nonpolar using our fun classroom quiz game Quizalize. Then, add octets to the outer atom and extra electrons to the central atom. There is a square plane containing the central atom and four of the atoms. As discussed, here there are 24 electrons. An example is BrF5, whose molecular geometry is square pyramidal (Table 7.3). To draw a Lewis Structure, first of all, add electrons and draw the connectivities. If we check the formal charges for the Boron Trifluoride Lewis structure, we will find that they are zero even though Boron only had six valence electrons. The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of 8 of the. We review their content and use your feedback to keep the quality high. The molecule will have a total of 36 valence electrons - 7 from bromine, 7 from each of the four fluorine atoms, and one extra electron to give the ion the -1 charge. What is the electron-pair geometry for Br in BrF5 What is the electron-pair geometry for Cl in ClF3 What is the the shape (molecular geometry) of ClF3 Who are the experts Experts are tested by Chegg as specialists in their subject area. It requires six valence electrons in its outer shell. Start from the Lewis structure of the tetrafluoroborate ion, BrF4(-). Before completing the octets, don’t forget to determine how many valence electrons there in Boron Trifluoride and place them accordingly.īoron will be at the center of the structure because of being least electronegative. BF3 has a total of 24 valence electrons, which we have to set around the central atom. To know about BF3 Lewis structure, we have to calculate the total number of valence electrons for the BF3 molecule.


 0 kommentar(er)
0 kommentar(er)
